- Note
- Published:
Investigation on the hydrogen abstraction from methyl glucoside by active oxygen species under oxygen delignification conditions IV: appearance of kinetic isotope effect in the reaction between methyl glucoside and deuterated methyl glucoside
Journal of Wood Science volume 58, pages 563–569 (2012)
Abstract
In our previous studies, a clear kinetic isotope effect was observed when a pair of carbohydrate model compounds, methyl β-d-glucopyranoside (MGPβ) and a deuterated MGPβ labeled at the anomeric (methyl β-d-(1-2H)glucopyranoside) or C-2 position (methyl β-d-(2-2H)glucopyranoside), was reacted with active oxygen species (AOS) generated by reactions between O2 and a phenolic compound, 2,4,6-trimethylphenol (TMPh), under oxygen delignification conditions. These results indicate that the AOS abstract the anomeric and C-2 hydrogens of MGPβ. Contrarily, no clear kinetic isotope effects were observed when AOS were generated by reactions between O2 and another phenolic compound, 4-hydroxy-3-methoxybenzyl alcohol (vanillyl alcohol, Valc), and hence, the abstraction of the anomeric and C-2 hydrogens by the AOS was not confirmed. In this study, a pair of MGPβ and the deuterated MGPβ labeled at all the positions, (2H3)methyl β-d-(1,2,3,4,5,6,6-2H7)glucopyranoside, was reacted with AOS generated from TMPh or Valc under oxygen delignification conditions to further examine the appearance of a kinetic isotope effect. A clear kinetic isotope effect was observed when either of TMPh or Valc was the origin of AOS, indicating that some AOS abstract at least a hydrogen of MGPβ in either case. The results are further discussed focusing on the type of AOS generated from TMPh and Valc.
Introduction
It is still a serious problem that oxygen delignification is accompanied by severe damage to carbohydrates. To overcome this problem, it is necessary to develop a deeper understanding of the fundamental chemistry of oxygen delignification. It is known that the degradation of carbohydrates in oxygen delignification is not caused by direct attack of O2 but by active oxygen species (AOS), which are mainly generated by reactions between O2 and phenolic units in lignin [1–5].
We investigated as to which hydrogen of a carbohydrate model compound, methyl β-d-glucopyranoside (MGPβ, Fig. 1), was preferentially abstracted by AOS under oxygen delignification conditions [6–8]. A pair of carbohydrate model compounds, MGPβ and a deuterated MGPβ labeled at the anomeric position (methyl β-d-(1-2H)glucopyranoside, MGPβ-1D, Fig. 1) or the C-2 position (methyl β-d-(2-2H)glucopyranoside, MGPβ-2D, Fig. 1), was treated under oxygen delignification conditions in the presence of a phenolic compound, 2,4,6-trimethylphenol (TMPh, Fig. 1) or 4-hydroxy-3-methoxybenzyl alcohol (vanillyl alcohol, Valc, Fig. 1). The carbohydrate model compounds were not degraded by O2 but AOS, and various types of AOS were generated by reactions between O2 and TMPh or Valc. When a pair of MGPβ and MGPβ-1D or MGPβ-2D was treated together with TMPh, the degradation of MGPβ was greater than that of MGPβ-1D or MGPβ-2D, and hence a kinetic isotope effect was clearly observed in each case [6, 7]. On the other hand, the degradation of MGPβ-1D or MGPβ-2D was not clearly different from that of MGPβ, when each of these pairs was treated together with Valc [8]. When each of these pairs was also treated with alkaline H2O2 without the presence of TMPh or Valc, the degradation of MGPβ-1D or MGPβ-2D was not clearly different from that of MGPβ [6–8]. In this system, it was assumed that only oxyl anion radical ( ), the conjugate base of hydroxyl radical (HO·, pK
a = 11.9), is the only AOS that is reactive with MGPβ. Thus, no clear kinetic isotope effects were observed, when the origins of AOS were Valc and H2O2. As a summary, the appearance of a kinetic isotope effect in our previous studies [6–8] is listed in Table 1. These results clearly indicate that the anomeric and C-2 hydrogens of MGPβ are abstracted by some AOS particular to those generated only from TMPh. The results can also apparently imply that all the AOS generated from Valc including
), the conjugate base of hydroxyl radical (HO·, pK
a = 11.9), is the only AOS that is reactive with MGPβ. Thus, no clear kinetic isotope effects were observed, when the origins of AOS were Valc and H2O2. As a summary, the appearance of a kinetic isotope effect in our previous studies [6–8] is listed in Table 1. These results clearly indicate that the anomeric and C-2 hydrogens of MGPβ are abstracted by some AOS particular to those generated only from TMPh. The results can also apparently imply that all the AOS generated from Valc including  do not frequently abstract the anomeric and C-2 hydrogens of MGPβ.
do not frequently abstract the anomeric and C-2 hydrogens of MGPβ.
It may be plausible that no clear kinetic isotope effect will be observed between the degradations of MGPβ and a deuterated MGPβ labeled at another position, C-3, -4, -5, -6, or the aglycon when AOS are generated from Valc or H2O2. Because in this case the results obtained in using each of these deuterated MGPβ may not be sufficient to discuss the reactivity of AOS in spite of the difficult preparations of these deuterated MGPβ compounds, the deuterated MGPβ labeled at all the positions, (2H3)methyl β-d-(1,2,3,4,5,6,6-2H7)glucopyranoside (MGPβ-allD, Fig. 1) should be suitable for a compound in the next step. In this study, a pair of MGPβ and MGPβ-allD was treated together with TMPh or Valc under oxygen delignification conditions, and the difference in the degradations between MGPβ and MGPβ-allD was examined to discuss the appearance of a kinetic isotope effect observed in this and our preceding studies [6–8]. The pair was also treated with alkaline H2O2 without the presence of TMPh or Valc to restrict AOS that are reactive with MGPβ and MGPβ-allD only as  .
.
Materials and methods
Materials
MGPβ and TMPh were purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan), and recrystallized from a mixture of EtOH/n-C6H14 and n-C6H14, respectively. Valc was purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan), and recrystallized from a mixture of EtOH/n-C6H14. MGPβ-allD was synthesized from d-(1,2,3,4,5,6,6-2H7)glucose (Taiyo Nippon Sanso, Co., Ltd., Tokyo, Japan). It was dissolved in methanol containing H2SO4 at room temperature for 5 days, and MGPβ-allD produced was separated from the corresponding α-anomer using preparative thin layer chromatography after converting them to the perbenzoylated derivatives. When the method of Koenigs and Knorr [9], which is the most common for the synthesis of MGPβ, was preliminarily applied, both MGPβ-allD and the corresponding α-anomer formed, so that the above-described method was used. Semiconductor grades (99.99+ %) of NaOH, FeCl3 and CD3OD (Sigma-Aldrich Japan K. K., Tokyo, Japan) were used without further purification. A H2O2 solution (30 %) containing no stabilizer and all the other chemicals were purchased from Wako Pure Chemical Industries, Ltd., and used without further purification. Ultra-high-purity water produced by reverse osmosis filtration (Puric-Z, Organo Co., Tokyo, Japan) was used in all the experiments.
No signal was observed in the 1H-NMR (JNM-A500, 500 MHz, JEOL Ltd., Tokyo, Japan) spectrum of MGPβ-allD, when D2O was used as the solvent. The 13C-NMR (JNM-A500, 500 MHz, JEOL Ltd.) spectrum of MGPβ (solvent: D2O) is shown in Fig. 2(a): δ 57.7 (–OCH3), 61.3 (C6), 70.1 (C4), 73.5 (C2), 76.2–76.4 (C3 and C5), 103.7 (anomeric). The 13C-NMR spectrum of MGPβ-allD (solvent: D2O) is shown in Fig. 2(b): δ 56.7–57.4 (–OCD3), 60.2–60.9 (C6), 69.3–70.0 (C4), 72.8–73.5 (C2), 75.4–76.5 (C3 and C5), 102.9–103.6 (anomeric).
Oxygen-alkali treatment
The reaction solution (300 ml) contained NaOH (0.5 mol/l), FeCl3 (0.36 mmol/l), the phenolic compound, TMPh or Valc (0 or 9.0 mmol/l), and a pair of the carbohydrate model compounds, MGPβ and MGPβ-allD (4.0 mmol/l each). The solution was transferred into a Teflon-coated stainless steel vessel (Taiatsu Techno® Co., Tokyo, Japan), and O2 was pressurized to 1.1 MPa. The vessel was heated to 95 °C for 10 min, which was maintained for 360 min with stirring. Reaction time was defined as 0 when the temperature reached 95 °C. At prescribed times, a portion of the solution was withdrawn for the quantification of the model compounds.
Alkaline hydrogen peroxide treatment
The reaction solution (29.8 ml) contained NaOH, FeCl3, MGPβ, and MGPβ-allD. The solution was transferred into a Teflon vessel (50 ml) and heated to 95 °C in a bath of a saturated NaCl solution. A H2O2 solution (30 %, 0.2 ml) was added to the vessel, and the reaction time at this moment was defined as 0. The concentrations of all the additives were the same as those in the aforementioned oxygen-alkali treatment after the addition of the H2O2 solution. The initial concentration of H2O2 was 58.8 mmol/l. At prescribed times, a portion of the reaction solution was withdrawn for the quantification of the model compounds, and reacted with NaBH4 at room temperature for 30 min to quench any possibly remaining H2O2.
Quantification of residual model compounds
For the quantification of the model compounds, all the work-up and analytical procedures were exactly the same as those described in our previous reports [6–8].
In the GC analysis of MGPβ and MGPβ-allD, these compounds showed two distinct peaks, although a common non-polar capillary column was used. This phenomenon was different from those observed in the analyses of MGPβ, MGPβ-1D, and MGPβ-2D, where GC/MS was also used for the quantification [6–8].
Results and discussion
The novel features of the reaction system employed in this study and the grounds to apply deuterated MGPβ compounds were introduced in our previous reports [6–8].
The carbohydrate model compounds used in this study, MGPβ and MGPβ-allD, were quite stable in the oxygen-alkali treatment without the addition of TMPh or Valc, which indicates that these compounds are not degraded by alkaline-induced reactions and the attack of O2. Therefore, the degradation of these compounds observed in the oxygen-alkali treatment of this study is caused by the attack of AOS generated by reactions between O2 and the phenolic compound, TMPh or Valc. The degradation observed in the alkaline H2O2 treatment of this study is caused by the attack of  produced by the decomposition of H2O2.
produced by the decomposition of H2O2.
Various types of AOS are generated by reactions between O2 and TMPh and between O2 and Valc. It should be noted that  is one of the AOS generated commonly from both TMPh and Valc under the conditions employed and not only
is one of the AOS generated commonly from both TMPh and Valc under the conditions employed and not only  but also many other AOS are generated from TMPh and Valc.
but also many other AOS are generated from TMPh and Valc.
In this and preceding studies, the carbohydrate model compounds are supposed to be degraded by the attack of AOS on the hydrogens of C–H bonds. Numerous previous studies on the fundamental chemistry of oxygen delignification support this assumption [1–21], although it cannot completely be ruled out that AOS primarily abstract the hydrogens of O–H bonds. Since it is currently impossible to examine this reaction mode, this mode is excluded from discussion in this and preceding papers.
Appearance of a kinetic isotope effect when the origin of AOS is Valc or H2O2
As described in the “Introduction”, the failure to observe a clear kinetic isotope effect in the reactions using Valc and H2O2 as the origins of AOS (Table 1) can apparently imply that all the AOS generated from Valc do not frequently abstract the anomeric and C-2 hydrogens of MGPβ and that some AOS including  abstract the other hydrogens. To further examine the reactivity of AOS toward hydrogens of MGPβ and discuss the appearance of a kinetic isotope effect, a pair of MGPβ and MGPβ-allD was treated together with Valc under oxygen delignification conditions. The pair was also subjected to the alkaline H2O2 treatment.
abstract the other hydrogens. To further examine the reactivity of AOS toward hydrogens of MGPβ and discuss the appearance of a kinetic isotope effect, a pair of MGPβ and MGPβ-allD was treated together with Valc under oxygen delignification conditions. The pair was also subjected to the alkaline H2O2 treatment.
Figure 3 shows the degradation of MGPβ and MGPβ-allD in two trials of the oxygen-alkali treatment using Valc as the origin of AOS. The experiments could be conducted only twice owing to the limited amount of d-(1,2,3,4,5,6,6-2H7)glucose available and the moderate yield of MGPβ-allD in the synthesis. Although the reproducibility of two trials was not very high, the most important result is that all the yields of MGPβ-allD were always higher than those of MGPβ at all the reaction times when these compounds were quantified.
The degradation of Valc was relatively fast and Valc disappeared from the reaction system at a reaction time of about 60 min (data not shown). The degradation of the carbohydrate model compounds was fast in the period when Valc still existed in the reaction system, and became moderate after the disappearance of Valc.
It is rational to assume that  is the only AOS that degrades the carbohydrate model compounds in the alkaline H2O2 treatment of this study, which was described in our previous reports [6, 7]. Figure 4 shows the degradation of MGPβ and MGPβ-allD in the alkaline H2O2 treatment. The experiments could be conducted only once owing to the limited amount of d-(1,2,3,4,5,6,6-2H7)glucose available and the moderate yield of MGPβ-allD in the synthesis. Although the yields of the compounds were not very stable after a reaction time of after 5 min, the most important result is that all the yields of MGPβ-allD were always higher than those of MGPβ at all the reaction times when these compounds were quantified.
is the only AOS that degrades the carbohydrate model compounds in the alkaline H2O2 treatment of this study, which was described in our previous reports [6, 7]. Figure 4 shows the degradation of MGPβ and MGPβ-allD in the alkaline H2O2 treatment. The experiments could be conducted only once owing to the limited amount of d-(1,2,3,4,5,6,6-2H7)glucose available and the moderate yield of MGPβ-allD in the synthesis. Although the yields of the compounds were not very stable after a reaction time of after 5 min, the most important result is that all the yields of MGPβ-allD were always higher than those of MGPβ at all the reaction times when these compounds were quantified.
The decomposition of H2O2 was significantly fast, and it disappeared from the reaction system at a reaction time of less than 5 min (data not shown). Therefore, the degradation of the carbohydrate model compounds occurred only in the initial phase.
As described above, the degradation of MGPβ was greater than that of MGPβ-allD during the whole reaction period in both the oxygen-alkali and alkaline H2O2 treatments, and hence, kinetic isotope effects were clearly observed in both cases. These results confirmed that some AOS generated from Valc including  abstract at least a hydrogen of MGPβ. The results obtained in this and our previous studies [6–8] can apparently imply that no AOS generated from Valc frequently abstract the anomeric and C-2 hydrogens of MGPβ and some AOS abstract at least a hydrogen at the positions other than the anomeric and C-2. This implication is based on the assumption that a kinetic isotope effect is clearly observed whenever an AOS abstracts a target hydrogen. If this is not the case, however, the obtained results can show that any hydrogen of MGPβ is abstracted by an AOS generated from Valc including
abstract at least a hydrogen of MGPβ. The results obtained in this and our previous studies [6–8] can apparently imply that no AOS generated from Valc frequently abstract the anomeric and C-2 hydrogens of MGPβ and some AOS abstract at least a hydrogen at the positions other than the anomeric and C-2. This implication is based on the assumption that a kinetic isotope effect is clearly observed whenever an AOS abstracts a target hydrogen. If this is not the case, however, the obtained results can show that any hydrogen of MGPβ is abstracted by an AOS generated from Valc including  without showing a clear kinetic isotope effect but kinetic isotope effect becomes observable accompanying the increase of the number of labeled deuteriums owing to the more frequent abstraction of deuterium by AOS. The dependence of the appearance of a kinetic isotope effect on the number of labeled deuteriums should be common, because opportunity for abstracting deuterium is much more frequent in the reaction of AOS with MGPβ-allD, which possesses ten deuteriums, than in the reaction of AOS with MGPβ-1D or MGPβ-2D, each of which possesses only one deuterium. If the above-described assumption on the observation of kinetic isotope effect is not either valid, it may also be possible that some AOS abstract the anomeric and C-2 hydrogens without showing clear kinetic isotope effects and contrarily kinetic isotope effects are observed when these AOS abstract the other hydrogens.
without showing a clear kinetic isotope effect but kinetic isotope effect becomes observable accompanying the increase of the number of labeled deuteriums owing to the more frequent abstraction of deuterium by AOS. The dependence of the appearance of a kinetic isotope effect on the number of labeled deuteriums should be common, because opportunity for abstracting deuterium is much more frequent in the reaction of AOS with MGPβ-allD, which possesses ten deuteriums, than in the reaction of AOS with MGPβ-1D or MGPβ-2D, each of which possesses only one deuterium. If the above-described assumption on the observation of kinetic isotope effect is not either valid, it may also be possible that some AOS abstract the anomeric and C-2 hydrogens without showing clear kinetic isotope effects and contrarily kinetic isotope effects are observed when these AOS abstract the other hydrogens.
To further consider the above-described possibilities, it is necessary to examine whether or not a clear kinetic isotope effect is observed when a pair of carbohydrate model compounds, MGPβ and MGPβ-3D, MGPβ and MGPβ-4D, MGPβ and MGPβ-5D, MGPβ and MGPβ-6D, or MGPβ and MGPβ-OMeD3, is treated together with Valc under oxygen delignification conditions and with the alkaline H2O2.
Appearance of a kinetic isotope effect when the origin of AOS is TMPh
Figure 5 shows the degradation of MGPβ and MGPβ-allD in two trials of the oxygen-alkali treatment using TMPh as the origin of AOS. The degradation of TMPh was relatively fast and it disappeared from the reaction system at a reaction time of about 45 min (data not shown).
As described in the “Introduction”, it was confirmed in our previous studies [6, 7] that the anomeric and C-2 hydrogens of MGPβ are abstracted by some AOS particular to those generated only from TMPh. In this study, the degradation of MGPβ was greater than that of MGPβ-allD during the whole reaction period in both trials, and hence, a kinetic isotope effect was clearly observed (Fig. 5). However, the difference in the degradations between MGPβ and MGPβ-allD was similar to those between MGPβ and MGPβ-1D and between MGPβ and MGPβ-2D observed in the reaction using TMPh as the origin of AOS in our previous studies [6, 7]. These results on AOS generated from TMPh seem to suggest that the AOS do not frequently abstract hydrogens at positions other than the anomeric and C-2. The comprehensive results obtained in this and our preceding studies [6–8] can apparently imply that AOS generated from TMPh preferentially abstract the anomeric and C-2 hydrogens and contrarily AOS produced from Valc including  do not frequently abstract these two hydrogens but hydrogens other than these two, although many kinds of AOS should be produced commonly from both TMPh and Valc. This implication is based on two assumptions: any deuterium abstraction is accompanied by observing a kinetic isotope effect. Kinetic isotope effect becomes more observable with increasing the number of labeled deuterium owing to the more frequent attack of AOS on deuterium. Because the above-described implication does not seem to be plausible, these two assumptions may not be valid.
do not frequently abstract these two hydrogens but hydrogens other than these two, although many kinds of AOS should be produced commonly from both TMPh and Valc. This implication is based on two assumptions: any deuterium abstraction is accompanied by observing a kinetic isotope effect. Kinetic isotope effect becomes more observable with increasing the number of labeled deuterium owing to the more frequent attack of AOS on deuterium. Because the above-described implication does not seem to be plausible, these two assumptions may not be valid.
To further consider these assumptions and discuss the reactivity of AOS in the TMPh system, it is necessary to examine whether or not a clear kinetic isotope effect is observed when a pair of carbohydrate model compounds, MGPβ and MGPβ-3D, MGPβ and MGPβ-4D, MGPβ and MGPβ-5D, MGPβ and MGPβ-6D, or MGPβ and MGPβ-OMeD3, is treated together with TMPh under oxygen delignification conditions.
Possible considerations on the type of AOS
In this and our preceding studies [6–8], at least some AOS particular to those originating only from TMPh are confirmed to abstract the anomeric and C-2 hydrogens of MGPβ, although various types of AOS are generated commonly from both TMPh and Valc. In the reaction system using TMPh as the origin of AOS, some AOS should structurally be similar to TMPh itself and the carbon skeleton of TMPh remains as its substructure. These AOS are possible candidates for those which are generated only in the reaction system using TMPh as the origin of AOS. The following text discusses what species can be proposed as these AOS.
A possible reaction scheme of a phenolic compound in the oxygen-alkali treatment is shown in Fig. 6. The one electron oxidation of TMPh or Valc by O2 affords 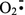 and the corresponding phenoxyl radical (I). The combination of
and the corresponding phenoxyl radical (I). The combination of 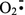 and (I) affords the peroxide (II). The ring cleavage reaction of (II) affords the relatively stable primary degradation product (III). The reductive cleavage of the peroxide bond in (II) by Fe2+ produces the important AOS, oxyl anion radical
and (I) affords the peroxide (II). The ring cleavage reaction of (II) affords the relatively stable primary degradation product (III). The reductive cleavage of the peroxide bond in (II) by Fe2+ produces the important AOS, oxyl anion radical  . The reductive cleavage can also produce one of the AOS, the alkoxyl radical (IV). The reductive cleavage is also promoted by
. The reductive cleavage can also produce one of the AOS, the alkoxyl radical (IV). The reductive cleavage is also promoted by 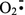 instead of Fe2+. Fe2+ can be produced by reduction of Fe3+ by a reducing agent, such as
instead of Fe2+. Fe2+ can be produced by reduction of Fe3+ by a reducing agent, such as 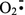 . The alkoxyl radical (IV) structurally consists of the carbon skeleton of the original phenolic compound, TMPh or Valc. The combination of the phenoxyl radical (I) and O2 can afford the peroxyl radical (V), which also structurally consists of the carbon skeleton of TMPh or Valc. The peroxyl radical (V) may be produced from the peroxide (II) by an oxidation, such as the oxidation by Fe3+. Thus, the alkoxyl (IV) and peroxyl (V) radicals originating from TMPh are possible candidates for the AOS that are not generated from Valc but only from TMPh and show clear kinetic isotope effects in the reactions with MGPβ and MGPβ-1D or with MGPβ and MGPβ-2D. It may not be plausible, however, that the reactivity of these radicals originating from TMPh is quite different from those generated from Valc. It should be pointed out that it has been discussed whether or not phenoxyl radicals (I) combine with O2 under oxygen delignification conditions. It was suggested in several previous reports that the combination reaction is unfavorable [14, 16–18, 21]. Because only model compounds consisting of a methoxyl-substituted aromatic ring were used in these previous reports, the combination reaction might be possible for the phenoxyl radical (I) originating from TMPh. If this is the case, the peroxyl radical (V) originating from TMPh is the most plausible candidate for the AOS that is produced only in the reaction system using TMPh as the origin of AOS and abstracts the anomeric and C2 hydrogens of MGPβ with showing clear kinetic isotope effects. On the other hand, the peroxyl radical (V) may not be generated from Valc on the basis of the suggestion of the previous reports [14, 16–18, 21].
. The alkoxyl radical (IV) structurally consists of the carbon skeleton of the original phenolic compound, TMPh or Valc. The combination of the phenoxyl radical (I) and O2 can afford the peroxyl radical (V), which also structurally consists of the carbon skeleton of TMPh or Valc. The peroxyl radical (V) may be produced from the peroxide (II) by an oxidation, such as the oxidation by Fe3+. Thus, the alkoxyl (IV) and peroxyl (V) radicals originating from TMPh are possible candidates for the AOS that are not generated from Valc but only from TMPh and show clear kinetic isotope effects in the reactions with MGPβ and MGPβ-1D or with MGPβ and MGPβ-2D. It may not be plausible, however, that the reactivity of these radicals originating from TMPh is quite different from those generated from Valc. It should be pointed out that it has been discussed whether or not phenoxyl radicals (I) combine with O2 under oxygen delignification conditions. It was suggested in several previous reports that the combination reaction is unfavorable [14, 16–18, 21]. Because only model compounds consisting of a methoxyl-substituted aromatic ring were used in these previous reports, the combination reaction might be possible for the phenoxyl radical (I) originating from TMPh. If this is the case, the peroxyl radical (V) originating from TMPh is the most plausible candidate for the AOS that is produced only in the reaction system using TMPh as the origin of AOS and abstracts the anomeric and C2 hydrogens of MGPβ with showing clear kinetic isotope effects. On the other hand, the peroxyl radical (V) may not be generated from Valc on the basis of the suggestion of the previous reports [14, 16–18, 21].
The magnitude of the appearance of a kinetic isotope effect can become a rough measure of the position of the transition state along the reaction coordinate [22]. When bond breaking is more or less than half complete at the transition state, the isotope effect is smaller and can disappear if the transition state is very reactant-like or very product-like [23]. The isotope effect is expected to be largest for the half that is complete at the transition state [24, 25]. It is considered that the transition state should be reactant-like and product-like in the hydrogen abstraction of AOS from MGPβ when the AOS have high and low reactivity, respectively. On the basis of the above description on the magnitude of the isotope effect, AOS with both high and low reactivity in the hydrogen abstraction from MGPβ may not show clear kinetic isotope effect in the oxygen-alkali treatment of a pair of the carbohydrate model compounds, MGPβ and a deuterated MGPβ. In this context, any AOS including  generated from Valc may have high or low reactivity toward the hydrogens of MGPβ, and consequently, no clear kinetic isotope effect may be observed in the oxygen-alkali treatment of a pair of MGPβ and a deuterated MGPβ, when the number of labeled deuterium on MGPβ is small. The reactivity of
generated from Valc may have high or low reactivity toward the hydrogens of MGPβ, and consequently, no clear kinetic isotope effect may be observed in the oxygen-alkali treatment of a pair of MGPβ and a deuterated MGPβ, when the number of labeled deuterium on MGPβ is small. The reactivity of  should be high. The reactivity of the AOS that is generated only from TMPh and shows a clear kinetic isotope effect in our previous studies [6, 7] may be moderate, and consequently, the transition state may lie in the middle along the reaction coordinates. This AOS has possibly been suggested above to be the peroxyl radical (V) of TMPh. The peroxyl radical (V) of Valc may not be produced because the combination reaction of the phenoxyl radical of Valc (I) with O2 is suggested to be unfavorable [14, 16–18, 21]. Thus, no AOS with moderate reactivity may be produced from Valc.
should be high. The reactivity of the AOS that is generated only from TMPh and shows a clear kinetic isotope effect in our previous studies [6, 7] may be moderate, and consequently, the transition state may lie in the middle along the reaction coordinates. This AOS has possibly been suggested above to be the peroxyl radical (V) of TMPh. The peroxyl radical (V) of Valc may not be produced because the combination reaction of the phenoxyl radical of Valc (I) with O2 is suggested to be unfavorable [14, 16–18, 21]. Thus, no AOS with moderate reactivity may be produced from Valc.
Conclusions
A kinetic isotope effect was clearly observed when a pair of MGPβ and MGPβ-allD was subjected to oxygen-alkali treatments using TMPh or VA as the origin of AOS under oxygen delignification conditions and to alkaline H2O2 treatments. It was confirmed that some AOS including  abstract hydrogens of MGPβ regardless of the origin of AOS, although no clear kinetic isotopes were observed in the degradations between MGPβ and MGPβ-1D or between MGPβ and MGPβ-2D in the oxygen-alkali treatments using Valc as the origin of AOS and the alkaline H2O2 treatments in our previous studies. Because these results cannot easily be understandable, the appearance of kinetic isotope effect should further be examined using other deuterated MGPβ compounds labeled at positions other than the anomeric and C-2.
abstract hydrogens of MGPβ regardless of the origin of AOS, although no clear kinetic isotopes were observed in the degradations between MGPβ and MGPβ-1D or between MGPβ and MGPβ-2D in the oxygen-alkali treatments using Valc as the origin of AOS and the alkaline H2O2 treatments in our previous studies. Because these results cannot easily be understandable, the appearance of kinetic isotope effect should further be examined using other deuterated MGPβ compounds labeled at positions other than the anomeric and C-2.
References
Ericsson B, Lindgren BO, Theander O (1971) Factors influencing the carbohydrate degradation under oxygen-alkali bleaching. Svensk Papperstidn 74(22):757–765
Gierer J, Imsgard F (1977) The reaction of lignins with oxygen and hydrogen peroxide in alkaline media. Svensk Papperstidn 80(16):510–518
Yokoyama T, Matsumoto Y, Yasumoto M, Meshitsuka G (1996) The role of peroxide species in the carbohydrate degradation during oxygen bleaching Part 2: Effect of oxygen pressure on the degradation of lignin and carbohydrate model compounds and on the reaction selectivity. J Pulp Pap Sci 22(5):J151–J154
Yokoyama T, Matsumoto Y, Meshitsuka G (1999) Reaction selectivity of active oxygen species in oxygen-alkali bleaching. J Wood Chem Technol 19(3):187–203
Yokoyama T, Matsumoto Y, Meshitsuka G (2005) Characterization of active oxygen species under oxygen-alkali bleaching conditions. Holzforschung 59(3):269–275
Konishi F, Yokoyama T, Matsumoto Y (2009) Investigation of hydrogen abstraction from methyl glucoside by active oxygen species under oxygen delignification conditions. Part 1: Study on the anomeric position. Holzforschung 63(1):52–60
Nakagawa A, Yokoyama T, Matsumoto Y (2012) Investigation of hydrogen abstraction from methyl glucoside by active oxygen species under oxygen delignification conditions. Part 2: Study on the C-2 position. J Wood Chem Technol 32(1):10–22
Yokoyama T, Nakagawa A, Konishi F, Matsumoto Y (2011) Investigation of hydrogen abstraction from methyl glucoside by active oxygen species under oxygen delignification conditions III: effect of the origin of active oxygen species. J Wood Sci 57(6):512–519
Koenigs W, Knorr E (1901) On some derivatives of dextrose and galactose. Chem Ber 34:957–981
Gierer J (1986) Chemistry of delignification. Part 2: Reactions of lignins during bleaching. Wood Sci Technol 20(1):1–33
Ek M, Gierer J, Jansbo K, Reitberger T (1989) Study on the selectivity of bleaching with oxygen-containing species. Holzforschung 43(6):391–396
Gierer J (1990) Basic principles of bleaching. Part 1: Cationic and radical processes. Holzforschung 44(5):387–394
Gierer J (1990) Basic principles of bleaching. Part 2: anionic processes. Holzforschung 44(6):395–400
Gierer J, Yang E, Reitberger T (1992) The reactions of hydroxyl radicals with aromatic rings in lignins, studied with creosol and 4-methylveratrol. Holzforschung 46(6):495–504
Gierer J, Jansbo K, Reitberger T (1993) Formation of hydroxyl radicals from hydrogen peroxide and their effect on bleaching of mechanical pulps. J Wood Chem Technol 13(4):561–581
Gierer J, Yang E, Reitberger T (1994) On the significance of the superoxide radical (
 /HO2·) in oxidative delignification, studied with 4-t-butylsyringol and 4-t-butylguaiacol. Holzforschung 48(5):405–414
/HO2·) in oxidative delignification, studied with 4-t-butylsyringol and 4-t-butylguaiacol. Holzforschung 48(5):405–414Gierer J, Yang E, Reitberger T (1996) The reactions of chromophores of the stilbene type with hydroxyl radical (HO·) and superoxide radical (
 /HO2·) part 1: The cleavage of the conjugated double bond. Holzforschung 50(4):342–352
/HO2·) part 1: The cleavage of the conjugated double bond. Holzforschung 50(4):342–352Gierer J, Yang E, Reitberger T (1996) The reactions of chromophores of the stilbene type with hydroxyl radical (HO·) and superoxide radical (
 /HO2·) part 2: Reactions other than cleavage of the conjugated double bond. Holzforschung 50(4):353–359
/HO2·) part 2: Reactions other than cleavage of the conjugated double bond. Holzforschung 50(4):353–359Gierer J (1997) Formation and involvement of superoxide (
 /HO2·) and hydroxyl (HO·) radicals in TCF bleaching processes: a review. Holzforschung 51(1):34–46
/HO2·) and hydroxyl (HO·) radicals in TCF bleaching processes: a review. Holzforschung 51(1):34–46Gierer J, Reitberger T, Yang EQ, Yoon BH (2001) Formation and involvement of radicals in oxygen delignification studied by the autoxidation of lignin and carbohydrate model compounds. J Wood Chem Technol 21(4):313–341
Sugimoto T, Morishita T, Matsumoto Y, Meshitsuka G (2000) Effect of oxygen pressure on the oxidation of syringyl alcohol initiated by manganese(III) acetate. Holzforschung 54(3):262–268
Lowry TH, Richardson KS (1987) 2.9 Isotope effects. In: Mechanism and theory in organic chemistry (3rd ed.). Harper & Row, HarperCollins Publishers, New York, pp 232–244
Carey FA, Sundberg RJ (2000) 4.5. Isotope effects. In: Advanced organic chemistry (4th ed.). Kluwer Academic/Plenum Publishers, New York, pp 222–224
Thornton ER (1962) Deductions from small primary deuterium isotope effects. J Org Chem 27(6):1943–1944
More O’Ferrall RA, Kouba J (1967) Model calculations of primary hydrogen isotope effects. J Chem Soc B 1967:985–990
Acknowledgments
The authors gratefully acknowledge financial support from the Japan Society for the Promotion of Science [Grant-in-Aid for Young Scientists (A), No. 20688007].
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nakagawa, A., Yokoyama, T. & Matsumoto, Y. Investigation on the hydrogen abstraction from methyl glucoside by active oxygen species under oxygen delignification conditions IV: appearance of kinetic isotope effect in the reaction between methyl glucoside and deuterated methyl glucoside. J Wood Sci 58, 563–569 (2012). https://doi.org/10.1007/s10086-012-1289-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10086-012-1289-z





